Abstract
Background: CD19-targeted chimeric antigen receptor T cell (CD19CART) therapy has been shown to be safe and effective for patients with relapsed/ refractory (r/r) diffuse large B cell lymphoma (DLBCL), mantle cell lymphoma (MCL) and follicular lymphoma (FL). However, there is limited data in other types of B-cell NHLs, especially those with central nervous system (CNS) involvement. Accumulating evidence suggests that using a memory T cell starting population to manufacture CD19CAR T cells improves the efficacy of therapy (Wang et al. Blood;127: 2980). Here we report the results of our Phase 1 trial evaluating CD19CART generated using naïve and stem cell memory (Tn/mem) T cells to treat adult patients with all types of r/r B-NHL (NCT02153580).
Methods: Trial eligibility included adult patients (≥18 years) diagnosed with CD19+ r/r B-NHL with Karnofsky performance score (KPS) of ≥70% and adequate organ function. Enrolled patients underwent leukapheresis and their autologous T cells were transduced with a hinge optimized CD19R(EQ)28z/EGFRt+ lentivirus containing a CD28 costimulatory domain and co-expressing truncated epidermal growth factor (EGFRt). All but 1 patient received a fludarabine/cyclophosphamide (Flu/Cy)-based lymphodepletion regimen prior to receiving CD19CART at 1 of 2 dose levels (DL): DL1=200 million (M) and DL2=600M CAR+ T cells. The first patient at DL1 received only Cy lymphodepletion but the decision to add Flu to all subsequent patients was then taken. Dose escalation was per the TEQR study design (Blanchard and Longmate. Contemp Clin Trials; 32;114). Primary objectives were safety and identification of the recommended Phase 2 cell dose (RP2D).
Results: Twenty-four participants (pts) with B-NHL underwent leukapheresis. Of these, 22 received their Tn/mem CD19CART products (9 at DL1, 13 at DL2) while 2 did not (1 went to transplant and 1 went to a compassionate use trial) [Table 1]. Median age was 60 years (range 41-81), 10 (45%) were female, 16 (73%) were white and 6 (27%) were Hispanic. Fifteen (68%) had a KPS score of ≥80% at enrollment. Median number of prior therapies was 5.5 (range, 1-15). Treated pts had high grade B-cell lymphoma (HGBCL)/DLBCL (7/22 including 2 Richter's transformation and 1 transformed (t)FL), primary CNS lymphoma (PCNSL) (5/22), MCL (4/22), FL (4/22) and Waldenstrom's macroglobulinemia (2/22). Two pts had active secondary CNS involvement (1 MCL and 1 DLBCL pt) at the time of CAR T cell infusion.
There were no dose limiting toxicities (DLTs) or any grade (gr) 3 or higher cytokine release syndrome (CRS) events, but 10 (45%) pts developed gr 2 CRS [Table 2]. Furthermore, 3 (14%) pts experienced gr 3 neurotoxicity that was possibly related to CD19CART cells (headache n=2; [dysphasia, encephalopathy, depressed level of consciousness], n=1). These 3 neurotoxicity events were in patients with PCNSL and were reversible. There was no grade 4 or higher neurotoxicity.
There were 12/22 (55%, 90%CI [35%,73%]) best objective responses (OR) of partial (PR) or complete response (CR): 4/9 at DL1 (2 PR, 2 CR) and 8/13 at DL2 (all CR). Notably, among the participants with PCNSL, 3/5 achieved a best OR of CR (DL1 n=1; DL2 n=2). At the time of this analysis, 14/22 pts had progressed, giving a median progression free survival of 5.3 months (90% (CI [2.4, NA]). Nine of 22 pts have died, all from disease progression. The median overall survival has not been reached after a median follow-up time of 21.5 months.
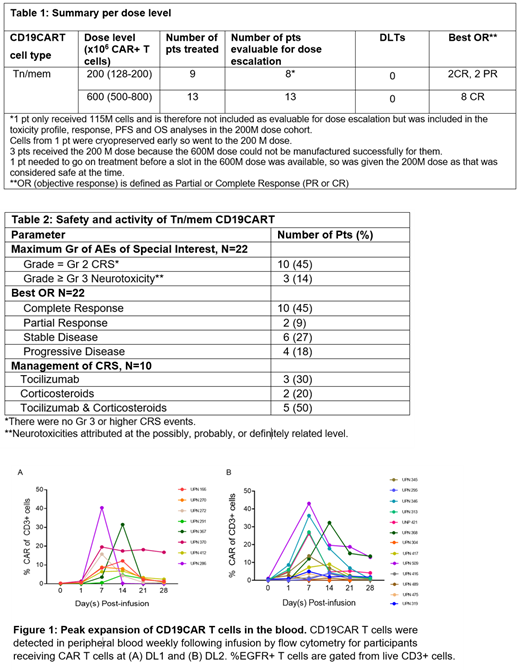
CAR T cell peak levels were 17.5%±4.5 (mean ± SE; DL1/200M, N=8) and 14.4%±3.9 (DL2/600M, N=13) (Figure 1 A, B). The 600M dose (DL2) was determined to be the RP2D based on the TEQR design, with the DLT rate of 0.0, 90% confidence limits (0.0, 0.21), as well as activity.
Conclusions: This clinical study provides evidence for the safety, feasibility and efficacy of Tn/mem-derived CD19CART preceded by Flu/Cy lymphodepletion for the treatment of r/r B-NHL. We plan to pursue this CAR T cell platform in future B-NHL studies, particularly for CNS lymphoma given the encouraging safety and efficacy of CD19CART in the 5 PCNSL patients.
Acknowledgements: The proposal for this protocol was developed at ASH CRTI workshop 2011-2012 by Tanya Siddiqi MD.
Siddiqi: Oncternal: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Research Funding; Janssen: Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Wang: Pepromene Bio, Inc.: Consultancy. Popplewell: Novartis: Other: Travel; Pfizer: Other: Travel; Hoffman La Roche: Other: Food. Herrera: ADC Therapeutics: Consultancy, Research Funding; Tubulis: Consultancy; Merck: Consultancy, Research Funding; Takeda: Consultancy; Gilead Sciences: Research Funding; Kite, a Gilead Company: Research Funding; AstraZeneca: Consultancy, Research Funding; Seagen: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Karyopharm: Consultancy. Budde: Mustang Bio, Inc: Research Funding; Merck, Inc: Research Funding; Amgen: Research Funding; BeiGene: Consultancy; IGM Biosciences: Research Funding; Roche: Consultancy; Gilead: Consultancy; Novartis: Consultancy; AstraZeneca: Research Funding. Nikolaenko: Rafael Pharmaceuticals: Research Funding; Pfizer: Research Funding. Brown: Mustang Bio: Patents & Royalties, Research Funding; Chimeric therapeutics: Patents & Royalties, Research Funding. Forman: Allogene: Consultancy; Lixte Biotechnology: Consultancy, Current holder of individual stocks in a privately-held company; Mustang Bio: Consultancy, Current holder of individual stocks in a privately-held company.
CD19-directed CAR T cell therapy manufactured at City of Hope being used for relapsed/refractory B-cell NHL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal